By: Marc Shomer, MD PhD
Board Certified Ophthalmologist
Clinical Instructor of Ophthalmology, Doheny Eye Institute
 First, what are GLP-1 medicines?
First, what are GLP-1 medicines?
Glucagon-like peptide-1 (GLP-1) is a natural hormone that helps control blood sugar, appetite, and digestion. GLP-1 receptor agonists are medicines that act like this hormone. They’re widely used for type 2 diabetes and obesity, and they can also lower risks of heart and kidney disease for many patients. Common brands include Ozempic® and Wegovy®(semaglutide) and Mounjaro® and Zepbound® (tirzepatide). Clinical guidance has increasingly favored these drugs for people with diabetes who also have cardiovascular or kidney risk. 1
What’s the headline concern?
Several observational studies have reported an association (not proof of cause) between semaglutide use and non-arteritic anterior ischemic optic neuropathy (NAION)—a sudden, usually painless loss of vision due to reduced blood flow to the optic nerve. At the same time, other lines of research suggest potential ocular benefits of GLP-1 drugs. 2 In addition, numerous studies have also identified potential benefits of these medications in ocular disease and whole body benefits (see below).
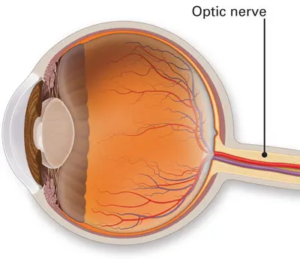 What is NAION?
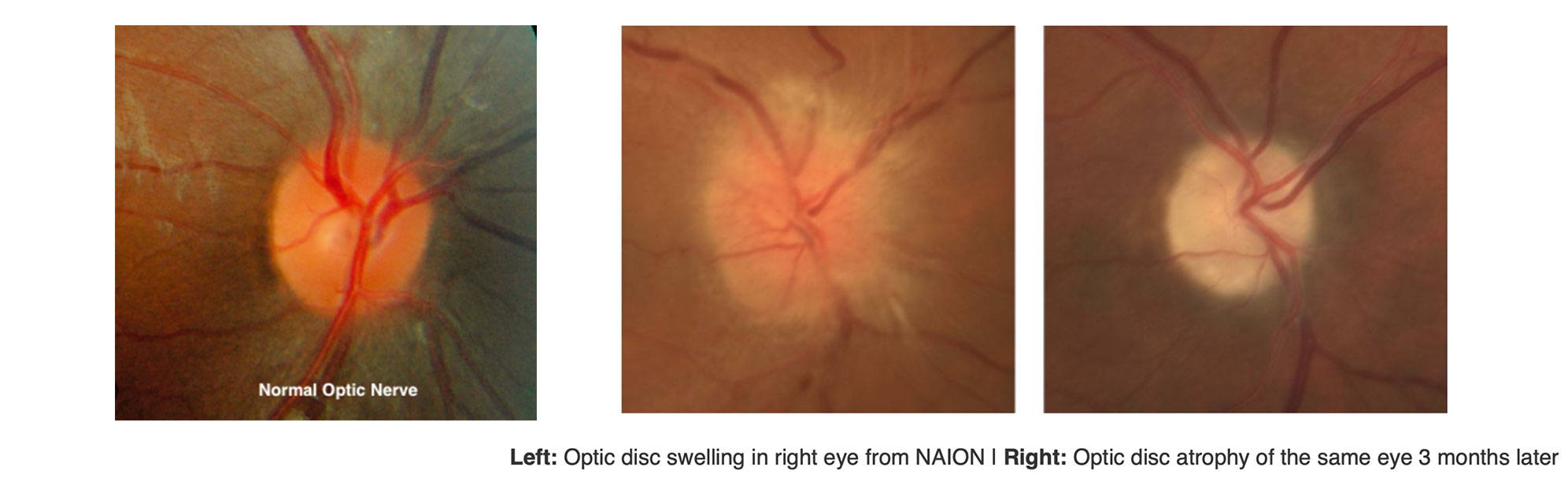
What is NAION?
NAION is essentially an “eye stroke” of the optic nerve which is the cable that carries signals from the eye to the brain. Blood flow through tiny vessels at the front of the optic nerve drops suddenly; part of the nerve doesn’t get enough blood flow and oxygen, and some nerve fibers die. People notice dim, blurry, or partially missing vision in one eye, typically without pain. NAION is more common after age 50 and in people with certain risk factors listed below. It occurs more often in eyes with a naturally crowded optic nerve called a disc at risk. 3
Common NAION risk factors
- Age over 50, hypertension, diabetes, high cholesterol or atherosclerosis, smoking (current or past), long-term kidney disease, anemia or acute blood loss, and clotting disorders. 3
- Obstructive sleep apnea (OSA) has a strong association with NAION. 4
- “Disc at risk” (small, crowded optic nerve cup) is frequently present in 60–80% of cases of NAION. 3
- Sudden dips in blood pressure (especially at night), peri-operative low blood pressure, and possibly use of erectile dysfunction (ED) medications called phosphodiesterase-5 inhibitors. 3
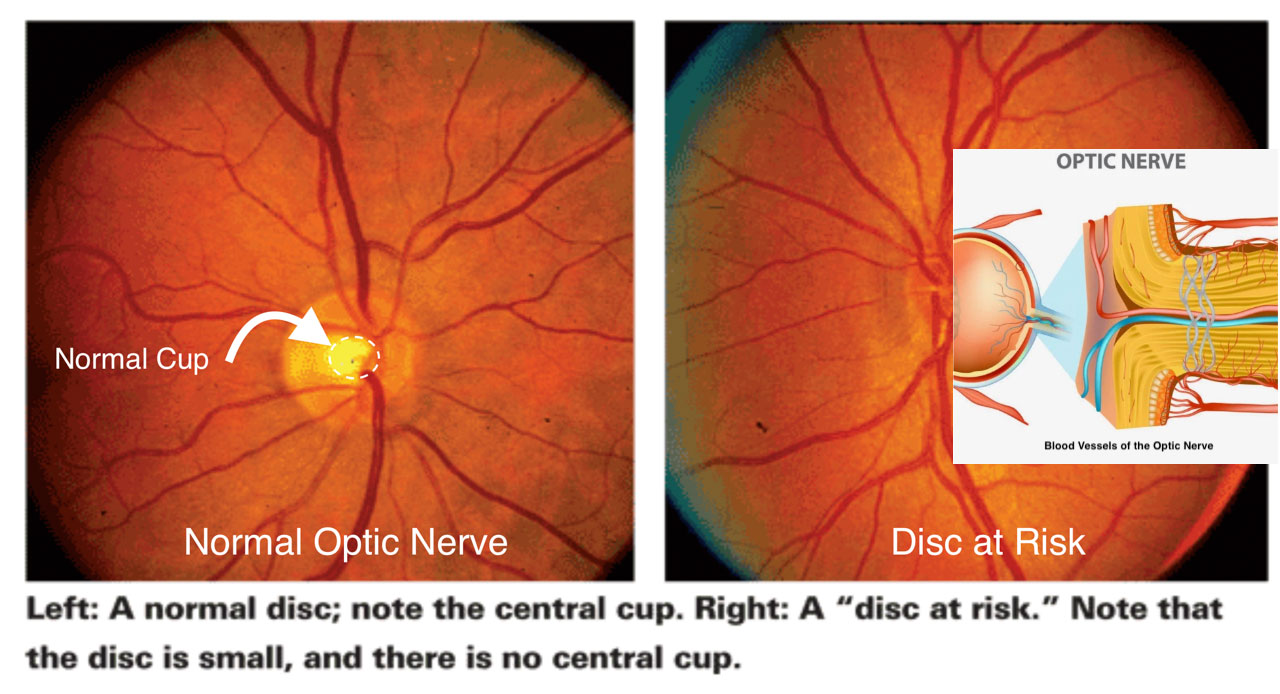
Why does blood flow stop in NAION?
In people with vascular risk factors, the inner lining of blood vessels (the endothelium) tends to be unhealthy, narrowing the vessel and making flow more fragile. Overnight, blood pressure normally dips; in a narrowed artery, that dip can be enough to briefly reduce flow to the optic nerve and trigger NAION which is one reason many patients notice symptoms upon waking. 3
What the associations of semaglutide use and risk of NAION?
- Mass Eye & Ear & Harvard study: Compared semaglutide users with patients on other therapies. They reported higher NAION rates among semaglutide users. Important caveat in this study is that the two groups differed in the rate of sleep apnea and obesity which could have skewed the data. It is also important to note that this study was an association and is not causation.
- Denmark national registry study: Type 2 diabetic patients on once-weekly semaglutide showed a higher 5-year risk of NAION versus non-users.
- US multicenter cohorts published in JAMA Ophthalmology, 2025: They observed about 2 times higher NAION risk in semaglutide users versus non-GLP-1 users. (Observational data; residual confounding likely.)
- Nordic cohort (Diabetes, Obesity & Metabolism, 2025): Ongoing/expanded analyses are refining absolute risk; early signals suggest low absolute rates overall.
Bottom line so far: signals of increased relative risk in some datasets, but absolute risk appears low, and causality hasn’t been proven. Better-controlled studies are in progress. 8
Why could there be an increased risk of NAION: Could blood-pressure changes play a role?
The studies show an association between semaglutide use and NAION, but not causation. People with diabetes and obesity, who are most likely to use GLP-1 medications, already have higher rates of sleep apnea and vascular disease, which are strong risk factors for NAION.
A 2024 study by Krumholz and colleagues found that starting GLP-1 medications can lower blood pressure within the first 24 weeks of use.1 If blood-pressure medications aren’t adjusted in time, this could lead to overly low blood pressure, especially at night, potentially increasing the risk of an optic-nerve “eye stroke” or NAION in susceptible individuals. Nighttime drop in blood pressure is already a known risk factor for NAION.
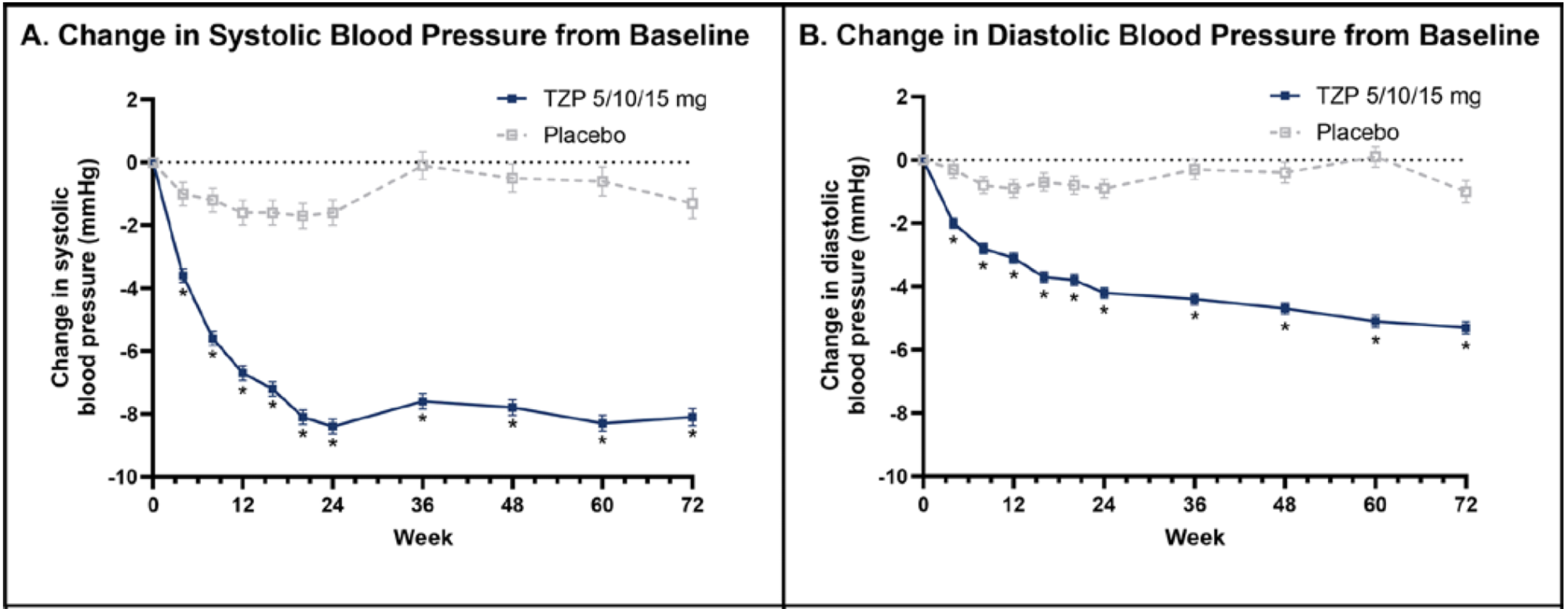
(SURMOUNT-1 randomized control trial, 2024)
Ongoing monitoring and medication adjustments by the treating physician are therefore important. This connection is still speculative and not proven. (Please see below for what you can personally do to help lower your risk of NAION. Talk to your doctor if taking your blood pressure medication before bedtime is a good idea considering blood pressure is already normally lower at night.)
Could GLP-1 drugs directly help the eye?
Every medication prescribed for all health conditions has potential benefits and risks. The appropriate use of any medication is to balance these factors to maximize benefit and minimize risk. The benefits of GLP-1 medications remain substantial. There is growing laboratory and early translational evidence that GLP-1 receptor agonists can reduce retinal inflammation, protect retinal ganglion cells, and stabilize the blood–retina barrier in diabetic and glaucoma models; topical (eye-drop) delivery has shown benefit in animal studies. Human receptor mapping suggests low but present GLP-1 receptor expression in the ganglion cell layer of normal eyes, with complex changes seen in advanced disease. Clinical trials are needed to confirm visual benefits. 9,10,11,12,13
In short, there are substantial benefits for overall health and eye health in patients using GLP-1 medications. Below is a summary of specific eye diseases that are likely caused or exacerbated by obesity and may be reduced by GLP-1 therapy.
- Diabetic retinopathy (DR):
Diabetes damages retinal blood vessels and is a leading cause of blindness in working-age adults. GLP-1 drugs show mixed signals in DR epidemiology, but several reviews and cohort studies do not demonstrate consistent DR worsening compared with other therapies when care is optimized. - Retinal vein occlusion (RVO):
Obesity and metabolic syndrome increase RVO risk in multiple populations, though effects may vary depending on diabetes status. - Idiopathic intracranial hypertension (IIH):
Rates are approximately 20 per 100,000 among obese women of child-bearing age, and weight loss is a core therapy. IIH is also a known cause of vision loss. - Glaucoma:
Multiple large datasets associate GLP-1 use with a lower risk of new glaucoma diagnoses, and early data suggest a small intraocular pressure–lowering effect. These findings remain observational.
GLP-1 medications and weight loss
GLP-1 medications also promote substantial weight loss, which has profound implications for eye health. Obesity is linked to the following mechanisms of ocular damage:
- Microvascular injury:
Chronic hyperglycemia, hypertension, and dyslipidemia damage the tiny blood vessels supplying the retina and optic nerve. - Inflammation and oxidative stress:
Adipose tissue releases cytokines and promotes oxidative damage, accelerating lens opacification and increasing macular and optic-nerve vulnerability. - Hemodynamic and pressure effects:
Obesity increases venous pressure, disrupts cerebrospinal fluid dynamics, and can raise intraocular pressure. - Sleep-disordered breathing:
Nocturnal hypoxia and blood-pressure dips in obstructive sleep apnea reduce optic-nerve perfusion and can worsen diabetic retinopathy.
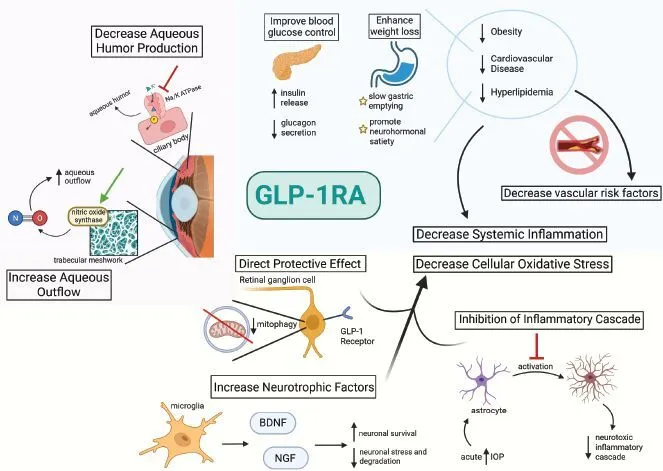
Chapman et al., Ophthalmology Management 2025
Practical steps to reduce your personal risk
- Ask your ophthalmologist about your optic nerve anatomy.
If your doctor sees a very small cup (“disc at risk”), discuss timing and monitoring when starting GLP-1 therapy. - Screen for sleep apnea (OSA) and use CPAP if prescribed.
OSA is strongly linked to NAION, and poor CPAP adherence increases fellow-eye risk. 21 - Tune up vascular risks.
Control diabetes, blood pressure, and cholesterol, and stop smoking. These factors are modifiable and within your control. 3 - Avoid nighttime blood pressure dips.
If you are losing weight or starting a GLP-1 medication, ask whether evening blood pressure medications should be adjusted, especially during the first 24 weeks. - Review bedtime blood pressure dosing.
Some blood pressure medications have peak effects soon after dosing, and blood pressure is already lower overnight; discuss whether bedtime dosing is appropriate for you. - Review medications that may add risk.
Erectile dysfunction medications (phosphodiesterase-5 inhibitors) and amiodarone have been implicated; review risks with your prescribers. 3,22 - Cataract surgery counseling.
NAION can rarely follow cataract surgery. Short-term risk is higher—especially if you have had NAION previously—though absolute risk remains low. 23,24
Summary of Benefits and Risks of GLP-1s
Benefits
- Gastrointestinal and metabolic:
Increased satiety and weight loss can reduce sleep apnea, improve blood pressure, and decrease cholesterol. - Pancreatic:
Improved glucose control. - Eye-related:
Decreased intraocular pressure, decreased macular degeneration risk, and potential reduction in many major obesity-associated eye diseases that are leading causes of blindness. - Neuronal protection and anti-inflammatory effects.
Risks
- Possible increased risk of a blinding condition (NAION), which remains uncommon, and some reports of increased diabetic retinopathy, though data are conflicting.
- Increased risk of a specific thyroid cancer (MEN-2), which is rare.
- Gastrointestinal side effects including reflux, constipation, diarrhea, and nausea (common).
- Gallbladder disease and pancreatitis (uncommon).
- Other risks have been reported.
In summary
Some observational studies report a possible link between GLP-1 drugs, especially semaglutide, and NAION. Absolute risk appears low, and causality has not been established.
GLP-1 therapies also provide major metabolic, cardiovascular, and renal benefits, and multiple studies suggest eye-specific benefits. For most patients, clinicians weigh these benefits against NAION risk and typically continue therapy with appropriate monitoring and risk-factor control.
NOTE:
This is not medical advice. Use this article as background for a discussion with your own clinicians, who know your personal medical history.
References
1. Krumholz HM, de Lemos JA, Sattar N, et al. Tirzepatide and blood pressure reduction: stratified analyses of the SURMOUNT-1 randomized controlled trial. Heart. 2024;110(19):1165–1171.
2. Niazi S, Sadda SR, Francis BA, et al. Association between glucagon-like peptide-1 receptor agonists and incident glaucoma. Ophthalmology. 2024;131(9):1056–1063.
3. Liu B, Li M, Wang T, et al. Risk factors for nonarteritic anterior ischemic optic neuropathy: a meta-analysis. Front Med. 2021;8:618353.
4. Salvetat ML, Zeppieri M, Tosoni C, et al. Non-arteritic anterior ischemic optic neuropathy (NA-AION): a review. Vision. 2023;7(4):72.
5. Hathaway JT, Shah MP, Hathaway DB, et al. Risk of nonarteritic anterior ischemic optic neuropathy in patients prescribed semaglutide. JAMA Ophthalmology. 2024;142(8):732–739.
6. Grauslund J, Taha AA, Molander LD, et al. Once-weekly semaglutide doubles the five-year risk of nonarteritic anterior ischemic optic neuropathy in a Danish cohort. Int J Retina Vitreous. 2024;10(1):97.
7. Hsu AY, Kuo H-T, Wang Y-H, et al. Semaglutide and NAION risk among patients with diabetes. JAMA Ophthalmology. 2025;143(5):400–407.
8. Simonsen E, Lund LC, Ernst MT, et al. Use of semaglutide and risk of NAION. Diabetes Obes Metab. 2025;27(6):3094–3103.
9–30. Additional references as listed in the original publication.
